The Sample Receipt Notification (SRN) process is how laboratories notify sample submitters of the receipt and status of samples. The SRN typically includes information such as the number of samples received, the overall state of the samples received, and details of any issues apparent in the samples received.
The SRN process provides sample submitters greater vision by tracking samples after they are shipped, but before analytical results are received. This information can be used to take decisive action as early as possible for any unforeseen issues with sample delivery. Such action may include scheduling resampling events, requesting retests from the laboratory, or notifying stakeholders of potential delays for result reporting.
The SRN module within EQuIS SPM tracks and documents laboratory receipt information and testing-related issues reported by laboratories. The SRN module contains a series of user entry forms and reports for viewing and editing data, as well as the SRN EDD format for loading data. The SRN module allows users to:
•Define groups of samples from a COC assigned to a laboratory for testing.
•Record COC and cooler information reported by a laboratory upon cooler reception.
•Record generic issues related to a group of samples received, as reported by the laboratory.
•Record issues related to specific samples within a group of samples received, as reported by the laboratory.
•Record issues related to specific tests associated with a group of samples received, as reported by the laboratory.
•Record issues related to unexpected containers within a group of samples received, as reported by the laboratory.
•Record delays in testing as reported by the laboratory.
•Record issues related to the SRN received by EQuIS data owners from the laboratory.
•Request resampling based on review of SRN information.
The SRN Module requires the SPM Schema be applied to the EQuIS Database. Once successfully applied, electronic SRN EDDs can be imported using the SRN.xsd format supplied with SPM (\Program Files\EarthSoft\SPM\Formats\SRN). The SRN data contained can be accessed via the SRN Module. The SRN Module is an EQuIS form, can be launched from SPM or EQuIS, and requires a SPM. dll to be present in the EQuIS\Forms folder.
Relationship Between SRN Module Items
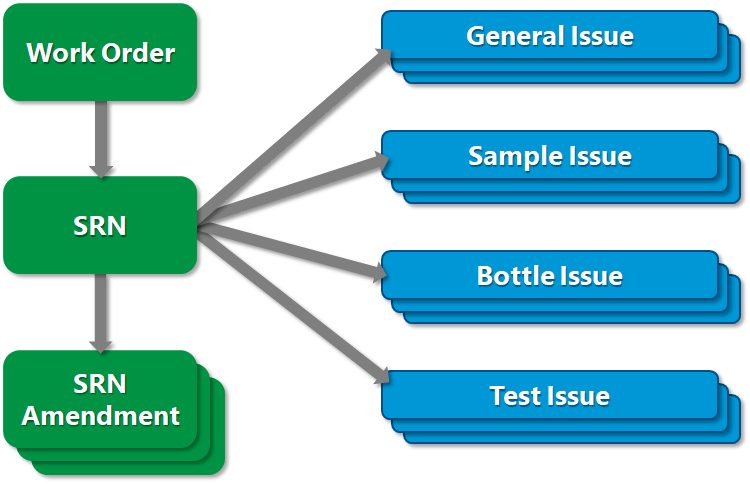
Using the SRN Manager, a work order is created for each COC to organize the SRNs associated with the COC. Specific SRNs can address general issues, sample issues, bottle issues, or test issues. SRN amendments can also be sent back to the laboratory.
SRN data can be received electronically from a laboratory (in the form of an SRN EDD) and processed automatically into the EQuIS database via Enterprise EDP or Professional EDP. Any electronically received data can be viewed and modified in the SRN Manager once loaded to the database. SRN data in another form, such as a PDF, can be entered manually using the SRN Manager in SPM. Depending on a project's workflow, SRNs may be created for every sample delivery group (SDG) / receipt or only in the event of a non-conformity reported by the laboratory.
Since the process of exchanging information between laboratories and EQuIS data owners can have multiple iterations for the same work order, an SRN entry is used to represent a data exchange event. One work order can have multiple SRN entries, with each entry defining the current list and state of samples and test issues. A work order is required to have a unique name, while each SRN entry is required to have a unique name and date/time combination. This facilitates the temporal tracking of any sample or test issues reported.
Refer to the SRN Manager topic to learn how to access, modify or create SRN data.